The UK’s lengthy and fraught CBD novel meals approval course of is displaying indicators of progress, with 5 new functions passing the Meals Requirements Company’s (FSA) security evaluation part for the reason that begin of 2025.
Nonetheless, the approvals have bolstered a contentious industry-wide debate over the FSA’s strict adherence to a 10mg acceptable day by day consumption (ADI) cap – a drastic discount from the earlier 70mg restrict that blindsided the {industry} in October 2023.
The 5 functions accredited up to now this 12 months superior roughly 850 merchandise, with greater than 830 of these from a joint utility submitted by TTS Pharma, Liverpool, and HERBL, California’s largest hashish distributor.
Arduous restrict on CBD consumption
Others functions advancing have been these filed by Brains Bioceutical, Mile Excessive Labs, cbdMD, and Bridge Farm Group. All 5 newly accredited functions conform to the 10mg ADI, a threshold that {industry} stakeholders have lengthy criticized as overly restrictive. Observers mentioned the approvals ship a robust sign from the FSA that functions proposing greater ADIs are unlikely to move the security assessment.
Trade group the Hashish Trades Affiliation has accused the FSA of misapplying the ADI as a binding cap slightly than advisory steering, arguing that the restrict doesn’t account for variations between CBD isolates, distillates, and full-spectrum extracts.
For the reason that ADI discount in October 2023, {industry} figures have warned that such a low threshold might render CBD merchandise ineffective, stifling market development and discouraging funding. In distinction, the European Industrial Hemp Affiliation (EIHA) has proposed a 17.5mg day by day restrict to European regulators, a extra average threshold that displays ongoing scientific evaluations.
Market uncertainty
Regardless of criticism over the ADI, the current approvals recommend the UK is transferring – albeit slowly – towards full CBD market regulation. Since CBD extracts have been designated as novel meals in January 2019, the FSA has struggled to handle an approval pipeline that originally noticed 12,000 product submissions. Up to now, roughly 5,000 merchandise have superior to the danger administration assessment stage.
Pending optimistic outcomes, the FSA and Meals Requirements Scotland will then suggest these merchandise to ministers throughout Nice Britain.
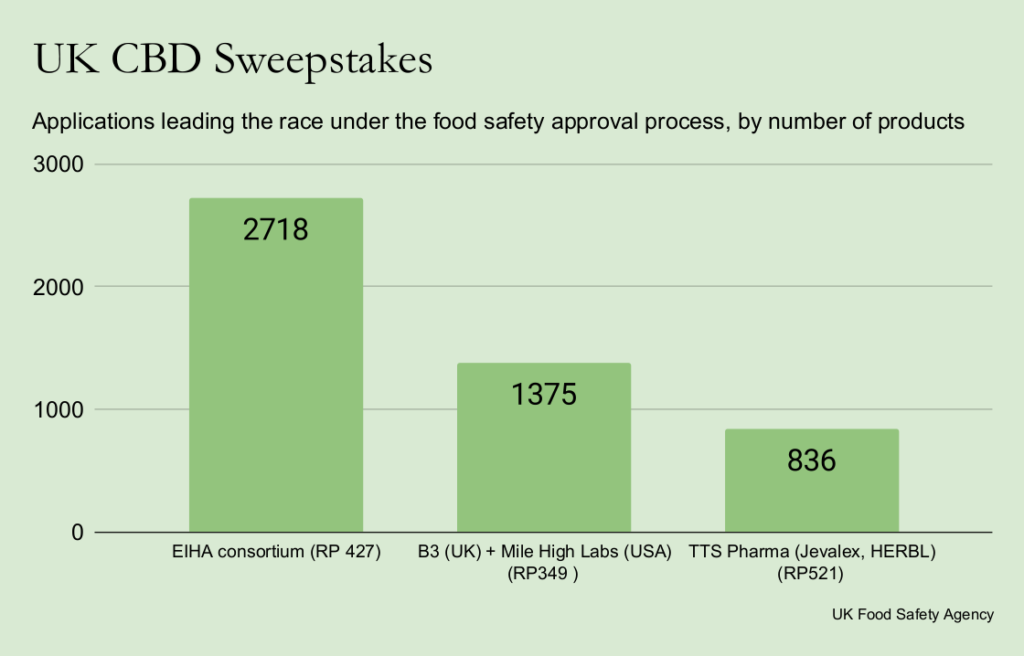
The newest approvals observe three granted in 2024, together with dossiers from Chanelle McCoy’s Pureis, Cannaray, and one from a consortium organized by the European Industrial Hemp Affiliation that continues to hold greater than 2,700 merchandise.
In accordance with the FSA’s most up-to-date report, the company expects to suggest a primary set of three product functions to UK ministers by mid-2025. If accredited, these would turn out to be the primary absolutely approved CBD merchandise legally bought within the UK market.
Alongside the brand new approvals, the FSA lately eliminated 102 merchandise from its public record of CBD merchandise allowed to stay in the marketplace pending full validation. Whereas some merchandise have been voluntarily withdrawn, others have been delisted with no clear clarification. Almost 600 have been faraway from the method completely to this point.
The EIHA consortium has one other 2,201 merchandise in a second utility for CBD distillates which stays on the first stage of the FSA’s assessment, “awaiting proof.”
An {industry} in limbo
The UK CBD market – valued at roughly $850 million – stays in a precarious place. Past the ADI debate, looming considerations over allowable THC ranges add additional uncertainty. The FSA, aligned with the House Workplace’s strict interpretation of the Misuse of Medicine Act, maintains that any detectable THC might render a product unlawful until it meets stringent Exempt Product Standards (EPC). This interpretation has already led to authorized disputes, such because the case of Jersey Hemp, which efficiently challenged a House Workplace resolution blocking its imports.
With an eight-week public session on CBD laws anticipated in early 2025, stakeholders anticipate additional clashes over each THC thresholds and the inflexible enforcement of the 10mg ADI. As of March 5, 2025, the FSA has not but initiated the session, a vital step within the FSA’s course of to suggest the primary set of CBD product functions.






